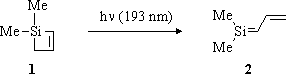
Martin Byloos - University of Ottawa
Placement: McMaster University
Supervisor: Dr. Willie Leigh
Current Position: Graduate Student, Department of Physics,
University of Ottawa
Far-UV Nanosecond Laser Flash Photolysis of 1,1-dimethyl-1-silacyclobut-2-ene
When 1,1-dimethyl-1-silacyclobut-2-ene (1) (Scheme I) is irradiated with far-UV light, it undergoes photochemical ring opening to yield 1,1-dimethyl-1,3-(1-sila)butadiene (2) (Scheme I), a reactive intermediate which has so far only been detected as its trapping product with methanol. It is the intent of this study to characterize the chemistry and the spectroscopy of 2 in hexane and acetonitrile solutions using Far-UV (193 nm) Nanosecond Laser Flash Photolysis, a new technique which has recently been developed in our lab.
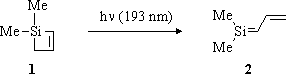
Scheme I
193-nm Laser flash photolysis of 1 yields a transient with an absorption maximum at 315 nm, and a lifetime of approximately 2-µs in hexane solution. It reacts rapidly with water and aliphatic alcohols, with rate constants of ~ 2 X 10^9 M¨¹s¨¹ in hexane solution. Based on these data, we assign the transient to 1,1-dimethyl-1,3-(1-sila)butadiene 2. It is thought that the unimolecular decay of 2 is by thermal ring closure to regenerate 1, in the absence of silene quenchers (water, aliphatic alcohols).
The project initially involved the synthesis of 1. The gas phase pyrolysis of diallyldimethylsilane (3; Scheme II) at 800°C produced 1 in roughly 30% yield. Compound 1 was purified by semi-preparative gas chromatography.
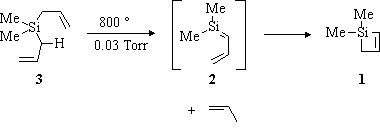
Scheme II
We are continuing our study of 2, and plan to measure Arrhenius parameters and solvent effects on the unimolecular decay process.
Back to: 1996 RISE Scholars
12-jul-96