Matthew Moran - McMaster University
Placement: University of British Columbia
Supervisor: Dr. John Scheffer
The Photochemistry of 2-Benzoyl-2-phenylbicyclo[3.1.0]hexane in the Solid State
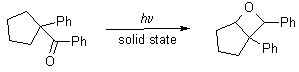
Photolysis of organic molecules, coupled with computational studies, has been integral in elucidating the reaction mechanisms that govern photochemical reactions. Of particular interest is solid state photolysis, as it negates any possible interference from solvents on the course of the reaction, especially when very reactive radical intermediates are formed. In keeping with such investigations, current efforts are being made to determine the mechanism by which oxetane formation occurs after photolysis of 1-benzenoyl-1-phenylcyclopentane. Previous work by Dr. Scheffer’s group (T. Kang et al., unpublished) shows that photolysis of 1-benzenoyl-1-phenylcyclopentane in the solid state (but not in solution) leads to the formation of 1,7 diphenyl-6-oxobicyclo[3.2.0]heptane.

Such a reaction is believed to happen through one of two mechanisms: (1) the Paterno-Büchi mechanism, which involves a-cleavage followed by hydrogen abstraction and [2+2] cycloaddition of the resulting aldehyde to 2-phenylcyclopent-1-ene, or (2) b-hydrogen abstraction by the carbonyl carbon followed by a radical ring closure.
In order to elucidate the mechanism of this solid state reaction, efforts are being made to synthesize 2-benzoyl-2-phenylbicyclo[3.1.0]hexane for similar solid state photolysis. It is thought that the addition of the cyclopropane ring will enable the system to undergo a classic radical rearrangement, forming a cyclohexenyl radical:
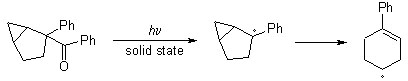
Such a rearrangement is made possible by the generation of a radical at the number two position of the bicyclic system after cleavage of the carbonyl group. As such, the formation of an oxetane to a six-membered ring would indicate that mechanism (1) was dominant. Otherwise, oxetane formation to the bicyclic system would suggest that mechanism (2) was preferred.
| Back to: | 2000 RISE Scholars RISE Home Page. |
15jul00-wjl