Sherman Hon - University of British Columbia
Placement: McMaster University
Supervisor: Dr. Willie Leigh
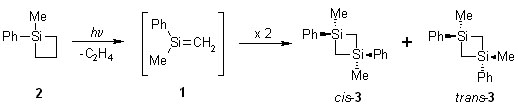
The Head-to-Tail [2+2]-Dimerization of Si=C Bonds
My project studies the kinetics of the dimerization of methylphenylsilene (1). There are 2 proposed mechanism for the reaction: 1) a concerted reaction; 2) a stepwise one with a .Si-C-Si-C. biradical as the intermediate. The silene is generated photochemically from methylphenylsilacyclobutane (2), which is synthesized by a Grignard reaction. The Arrhenius parameters for the dimerization process will be determined by flash photolysis of the silacyclobutane. The cis and trans isomers of the dimer (3) will then be photolysed separately to quantify the reactivity of the 1,4-biradical. This should give some insight towards the true mechanism of the reaction. Initial attempts to synthesize the dimer by photolysis of the silacyclobutane proved unsuccessful; we are trying to find an alternate source for the compound at this moment.

| Back to: | 2000 RISE Scholars RISE Home Page. |
15jul00-wjl